Our Research Interests
An overview of the research that goes on in the Anderson Lab
Research within the Anderson Lab has been closely integrated with many of the newest developments in both clinical practice and experimental science. Increasingly, the work has been orientated toward clinical translatability, both from the topical research perspective as well as the development of generalizable tools and frameworks that will facilitate the eventual development of robust clinical decision support tools that are based on data analysis and mathematical modeling. The research has culminated in the recent creation of a new Center of Excellence (CoE) in Evolutionary Therapy that has begun to take concrete steps toward implementing mathematical practice into the clinic. An Evolutionary Tumor Board, the first of its kind in the world, has begun meeting and is an active collaboration between numerous clinicians, biologists, and mathematical modelers that is using patient data to generate decision support using evolutionary theory and modeling. Much of the research in the Anderson Lab has been vital in supporting the activities of the CoE. Current and recent research has focused on several areas of interest: 1) the modeling of evolutionary therapies (such as adaptive therapy); 2) investigations of the complex dynamics between the immune system and cancer, including the effects of immunotherapies; 3) inquiries into the effect of physical space and spatial variation in tumors and the effects of this heterogeneity on treatments; and 4) research into tumor metabolism and the microenvironment. Much of this work is framed through the lens of homeostasis, its importance in normal tissue reglautation and the route to malignant transformation. Evolutionary therapy now has its own page and research in these other broad areas, which often can intersect, are described below.
Tissue Homeostasis
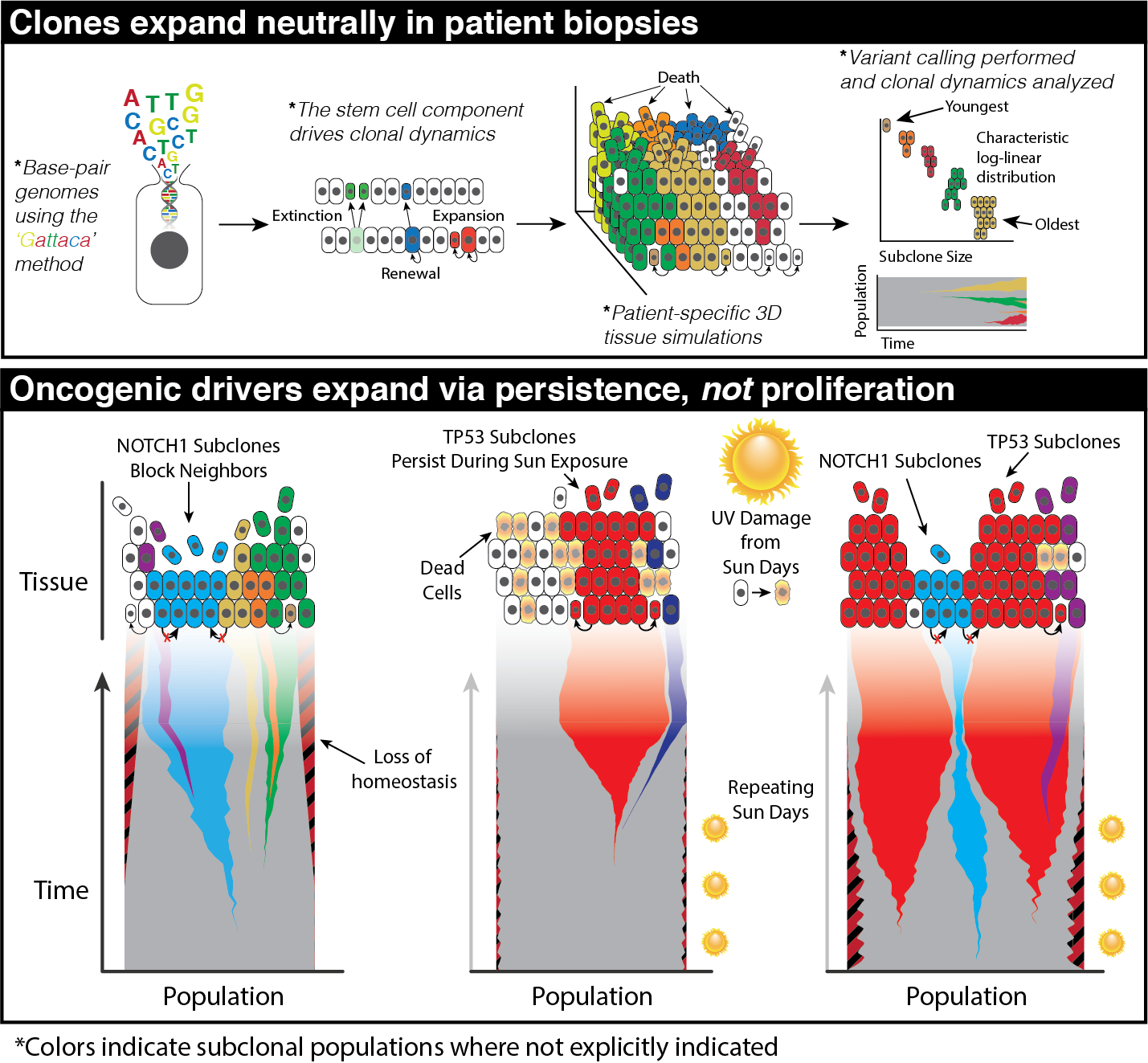 Cancer emerges from our homeostatic, normal tissue. This dynamic tissue ecosystem can favor the survival and proliferation of certain abnormal cellular phenotypes over their normal counterparts. This ecological viewpoint of cancer, which prioritizes an understanding of the normal underlying spatial constraints, structure, cross-talk, and cell populations, dictates that the goal is to return to or establish a new homeostasis. A number of studies have found oncogenic driver mutations present nearly ubiquitously within normal tissues, without evidence of a tumor. These studies call into question whether these clones carrying oncogenic driver mutations are larger than would be expected under neutral drift indicating they carry oncogenic potential alone or they exist within a constrained environment that may matter more than the mutations themselves.
Understanding the spatial constraints that homeostasis imposes and the environment where these mutations thrive is paramount to understanding the transformation from normal to cancer. This is difficult to do through experimental work alone; the integration of mechanistic modeling into research on homeostasis is proving valuable. However, this approach is also challenging, as data from normal tissues are rare due to ethical constraints and a lack of interest in preserving histology from normal biopsies. Furthermore, what data exists are often sparse.
Cancer emerges from our homeostatic, normal tissue. This dynamic tissue ecosystem can favor the survival and proliferation of certain abnormal cellular phenotypes over their normal counterparts. This ecological viewpoint of cancer, which prioritizes an understanding of the normal underlying spatial constraints, structure, cross-talk, and cell populations, dictates that the goal is to return to or establish a new homeostasis. A number of studies have found oncogenic driver mutations present nearly ubiquitously within normal tissues, without evidence of a tumor. These studies call into question whether these clones carrying oncogenic driver mutations are larger than would be expected under neutral drift indicating they carry oncogenic potential alone or they exist within a constrained environment that may matter more than the mutations themselves.
Understanding the spatial constraints that homeostasis imposes and the environment where these mutations thrive is paramount to understanding the transformation from normal to cancer. This is difficult to do through experimental work alone; the integration of mechanistic modeling into research on homeostasis is proving valuable. However, this approach is also challenging, as data from normal tissues are rare due to ethical constraints and a lack of interest in preserving histology from normal biopsies. Furthermore, what data exists are often sparse.
The constraint of homeostasis allows us to consider tissue using a first principles perspective. Primarily, we seek the minimum components from a systems approach that yields an emergent homeostatic model. In the case of most tissues, it can be deduced to simply balanced birth/death with a consistent stem cell number and genetic material that is heritable and mutable. While tissue-specific first principles exist that dictate structure, these structures dictate an equilibrium of balanced birth/death. Further, the spatial, hierarchical structure of cells within a homeostatic system is preserved within the cells and dictates how this hereditary information is maintained and stored. This information can be exploited to elucidate the molecular and evolutionary dynamics of structured tissues. A second area of homeostasis we investigate is the physical dynamics of normal cells and tissue in response to perturbations. Wound healing is a major component of homeostasis, and indeed cancer has often been characterized as “a wound that never heals”. The response to tissue damage includes regrowth of normal cells, rebuilding of the vascular network through angiogenesis and pruning, activity of the immune system, and secretion of numerous molecular signaling factors. The temporal dynamics of these various components typically follow a program of cleaning out damaged area followed by regrowth of the replacement tissue. These dynamics are thought to be critical in regards to tumor progression and growth. In one of our models, we examined the effect of vascular remodeling on cancer growth. The tumor in the simulation was embedded within a homeostatic self-repairing tissue that included normal cell turnover as well as angiogenesis and pruning. By only adjusting the vascular properties of the normal tissue, the resulting tumors evolved very differently, despite the fact that all the tumor cells in the different simulations were algorithmically identical. In other words, the starting phenotype of the tumor was not predictive of the outcome, but rather it was the homeostatic properties of the vasculature that determined the outcomes.
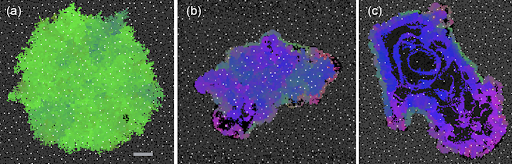 Here are three simulations with increasingly dysfunctional homeostatic properties for the vasculature. Each simulation started from identical smaller tumors. White dots represent blood vessels, with normal cells in gray. The tumor cells are colored according to their phenotype: green is the most benign and pink is the most invasive. All three panels are on the same scale, with the scale bar equal to 400 microns. (a) Results of a simulation with homeostatic vascular turnover. Here, the vessels are held essentially fixed for the entire simulation. The tumor took 5756 days to reach this size and is benign. (b) Results with sustained vascular turnover (constant pruning and remodeling), so that tumor perfusion is high but unstable. This tumor took 2721 days to reach this state and is just becoming invasive. (c) Results with limited angiogenesis and high turnover. This tumor took 1912 days to reach this size. Necrosis is now evident and the tumor is highly invasive. Overall, the results suggest that dysregulation of the vascular homeostasis can drastically change the trajectory of tumor phenotypes and resulting behavior of the cancer.
Here are three simulations with increasingly dysfunctional homeostatic properties for the vasculature. Each simulation started from identical smaller tumors. White dots represent blood vessels, with normal cells in gray. The tumor cells are colored according to their phenotype: green is the most benign and pink is the most invasive. All three panels are on the same scale, with the scale bar equal to 400 microns. (a) Results of a simulation with homeostatic vascular turnover. Here, the vessels are held essentially fixed for the entire simulation. The tumor took 5756 days to reach this size and is benign. (b) Results with sustained vascular turnover (constant pruning and remodeling), so that tumor perfusion is high but unstable. This tumor took 2721 days to reach this state and is just becoming invasive. (c) Results with limited angiogenesis and high turnover. This tumor took 1912 days to reach this size. Necrosis is now evident and the tumor is highly invasive. Overall, the results suggest that dysregulation of the vascular homeostasis can drastically change the trajectory of tumor phenotypes and resulting behavior of the cancer.
The importance of space in tumour evolution
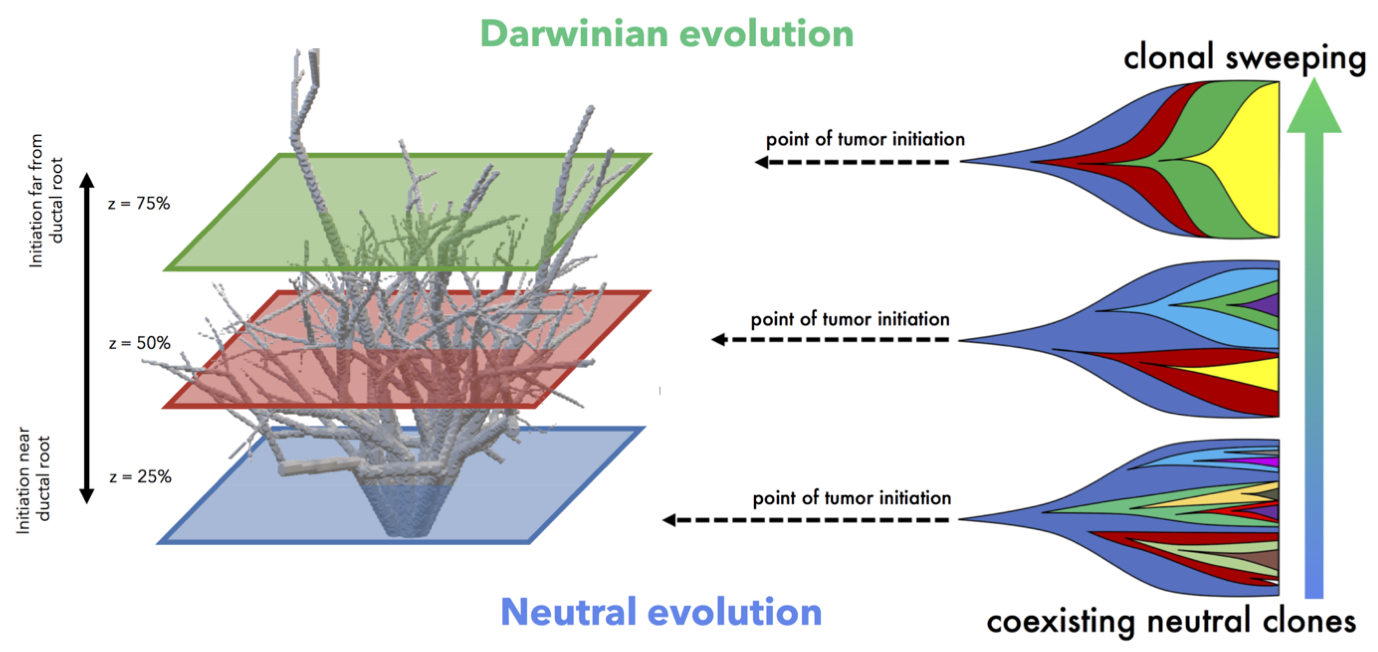 It’s clear that spatial constraints can no longer be ignored in the discussion of tissue evolution, normal or cancerous. One case where spatial constraints play a role is pre-malignant ductal carcinoma growth. Such ductal structures create selection pressures due to space limitations, enabling more fit clones to easily sweep through the domain. Using 3-dimensional an agent-based model to simulate accumulation of genetic diversity over time, we quantify the role of spatial constraints in ductal carcinoma in situ (West, 2019). Each tumor is is subject to varied constraints based on the point of tumor initiation, as well as limits on cellular mixing subject to the branching topology of the patient-specific breast ductal network. Tightly constrained tumors evolve in a Darwinian mode while less constrained tumors evolve in a neutral mode of evolution.
It’s clear that spatial constraints can no longer be ignored in the discussion of tissue evolution, normal or cancerous. One case where spatial constraints play a role is pre-malignant ductal carcinoma growth. Such ductal structures create selection pressures due to space limitations, enabling more fit clones to easily sweep through the domain. Using 3-dimensional an agent-based model to simulate accumulation of genetic diversity over time, we quantify the role of spatial constraints in ductal carcinoma in situ (West, 2019). Each tumor is is subject to varied constraints based on the point of tumor initiation, as well as limits on cellular mixing subject to the branching topology of the patient-specific breast ductal network. Tightly constrained tumors evolve in a Darwinian mode while less constrained tumors evolve in a neutral mode of evolution.
To illustrate the importance of spatial dynamics on cancer treatment, off-lattice, agent-based models have been used to investigate the spatial evolutionary dynamics of different treatment schedules on heterogeneous tumors. Space is the limiting factor, such that at carrying capacity, a cell will enter quiescence due to contact inhibition. Under two driving assumptions, 1) quiescent cells are unaffected by the drug and 2) resistant cells proliferate less quickly, our models show that spatial structure can affect the emergence of resistant phenotypes due to space limitation and limited drug perfusion (Gallaher, 2018).
Important transient behavior may also be lost without considering space. In another preprint, “Macrophage-mediated immunoediting drives ductal carcinoma evolution: Space is the game changer,” (Gatenbee, 2019) we have explicitly compared differences between non-spatial game theoretic models with spatially-explicit formulations. Agent-based models seeded from immunohistochemistry imaging data of ductal carcinomas, track the evolution of various immune escape strategies. The model includes macrophages, which are an important ‘public good’ in facilitating invasion, but lose importance after invasion when dynamics are mainly driven by stromal interactions. A spatial model allows for the emergence of a self-assorting engineer-pioneer dynamic of two immunoediting strategies (immunosuppressive and immunoresistant).
In each of these cases, the important result may have been lost without spatially-explicit models. In a non-spatial setting, fitter clones may rarely be constrained from expanding, with significant genetic hitch-hikers. Contrasting that to a spatial setting where many fitter clones may indeed exist – but are unable to undergo clonal expansion.
Tumour and immune interactions
 Cancer immunology has emerged as one of the most exciting and dynamic areas of translational cancer research. Harnessing the immune system has long been considered a potentially powerful method to combat cancer, but in most advanced tumors the immune response is largely a bystander. Mechanisms of immune evasion, tolerance, and escape have been poorly understood but generally accepted as reasons to write off immune involvement during treatment. What’s more, the immune system can even be co-opted by cancer cells to suppress inflammation, produce growth factors, and remodel tissue in order to favor tumor growth. Recently, vaccines, recombinant cells, and other immunostimulatory methods have demonstrated novel routes of overcoming these traditional roadblocks. Similarly, deeper investigations of cancer dynamics have demonstrated that the immune system may actually play a key role in many traditional cancer treatments.
Cancer immunology has emerged as one of the most exciting and dynamic areas of translational cancer research. Harnessing the immune system has long been considered a potentially powerful method to combat cancer, but in most advanced tumors the immune response is largely a bystander. Mechanisms of immune evasion, tolerance, and escape have been poorly understood but generally accepted as reasons to write off immune involvement during treatment. What’s more, the immune system can even be co-opted by cancer cells to suppress inflammation, produce growth factors, and remodel tissue in order to favor tumor growth. Recently, vaccines, recombinant cells, and other immunostimulatory methods have demonstrated novel routes of overcoming these traditional roadblocks. Similarly, deeper investigations of cancer dynamics have demonstrated that the immune system may actually play a key role in many traditional cancer treatments.
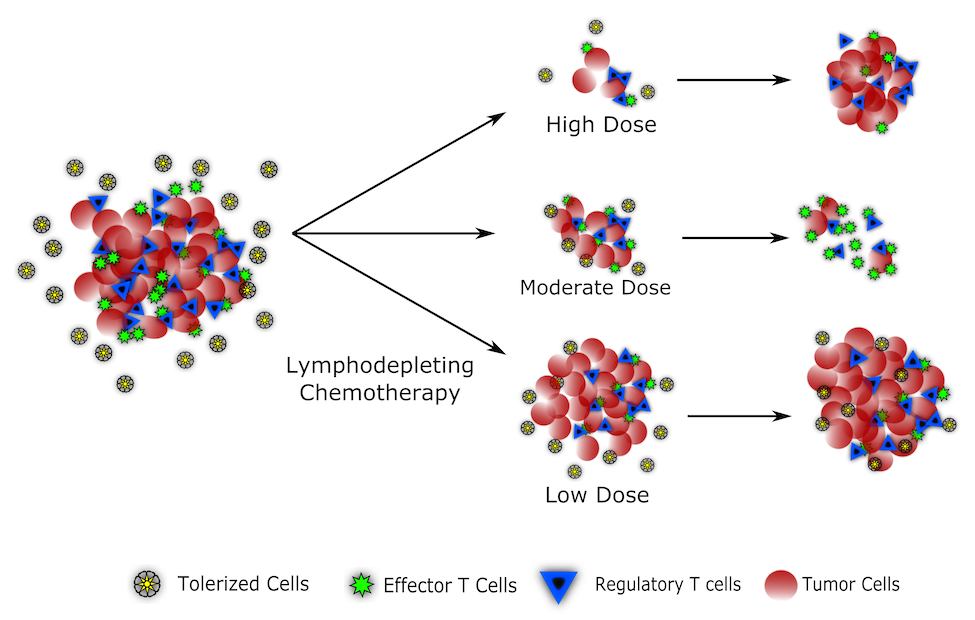
One major area of potentially unrealized benefits comes from synergizing immune responses with lymphodepleting chemotherapy. Chemotherapy has been notorious for its clinically disadvantageous impacts on the immune system. Neutropenia and increasing patient vulnerability have been one of the most important and detrimental side effects of multiple chemotherapeutic drugs. But the same lymphodepletion that renders patients susceptible to infection also clears out regulatory T cells and can resensitize the immune system to tumor antigens. Using mathematical modeling, we investigate this interplay and suggest that, contrary to popular wisdom, moderating chemotherapy doses within a ‘Goldilocks Window’ could lead to better treatment outcomes through maximizing both cytotoxicity and T-cell responses (Park 2019).
Tumors can avoid immune predation by evolving a combination of immunosuppressive and metabolic mechanisms. We are investigating how competition for glucose between tumor cells and T-cells provides tumors the opportunity to inactivate the immune attack via glucose starvation. Increased glycolytic activity in tumor cells, a commonly observed phenomenon in most aggressive cancers, increases the ease with which tumors deprive T-cells of the glucose needed to maintain cytotoxic function. Additionally, the acidic microenvironment in these highly glycolytic tumors also foments T-cell inactivation. We have also recently examined the construction of the immunosuppressive niche using game theory, branching process models, agent-based models, and ecological and spatial analyses of tumor biopsies (Gatenbee 2019a; Gatenbee 2019b; Gatenbee 2019c). Despite the different modeling approaches, all models arrive at the conclusion that construction of an immunosuppressive niche is a key event in tumor evolution. These predictions are consistent with our quantitative analysis of the tumor ecology in colorectal cancer, where we show that construction of the immunosuppressive niche begins at the earliest stages of transformation, and continues through progression, suggesting that transformation of an immunosuppressive tumor ecology into one that is pro-inflammatory may be a powerful form of immunotherapy.
Tumour metabolism and acid mediated invasion
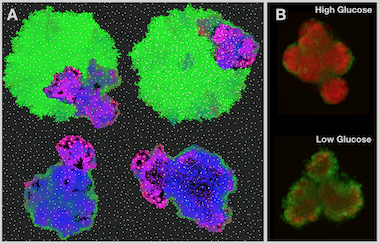 Cancers are characterised by uncontrolled proliferation, but cell division is expensive – 3 billion DNA base pairs and all the machinery to run a cell need to be made from scratch. This is made even more difficult in tumor tissue where resource supply, in particular oxygen, is often poor and waste products are cleared slowly - in short, conditions similar to a swamp! In order to adapt, cancer cells have to alter their metabolism, which in turn changes the resource composition of their environment. A key interest of our lab is to understand this interplay between cancer cell metabolism and tumor evolution, and how we may exploit it for treatment.
An important alteration in cancer cell metabolism is a focus on increased glycolysis, which allows energy production even in the absence of oxygen. Glycolysis is inefficient at producing energy, and as a by-product it acidifies the cell’s environment. Yet it is the main source of energy for many advanced cancers, even in the presence of oxygen - a phenomenon known as the Warburg effect. Together with the Gatenby and Gillies labs, we are exploring the hypothesis that the acid is an important driver of tumor invasion. Using a cellular automaton model we have shown that acid adaptation likely precedes the up-regulation of acid production (Robertson-Tessi, 2015), and that the presence of a glycolytic tumor phenotype is linked to tumor invasion in mouse models (Ibrahim-Hashim, 2017).
Another area in which tumor metabolism plays an important role is in the interaction between drug sensitive and drug resistant cells. Because resistant cells have to divert energy towards the resistance mechanism (e.g. production of drug pumps like p-glycoprotein) they have less energy available for proliferation. This hypothesis has motivated the idea of adaptive therapy, which has proven successful in pre-clinical experiments as well as pilot clinical trials (link to the adaptive therapy part of the site). But does a cost to resistance always exist? And if not, does it matter? To answer this question, we are investigating the role of such costs to resistance for the success of adaptive therapy, and are investigating the metabolism of resistant cells in the wet-lab.
Cancers are characterised by uncontrolled proliferation, but cell division is expensive – 3 billion DNA base pairs and all the machinery to run a cell need to be made from scratch. This is made even more difficult in tumor tissue where resource supply, in particular oxygen, is often poor and waste products are cleared slowly - in short, conditions similar to a swamp! In order to adapt, cancer cells have to alter their metabolism, which in turn changes the resource composition of their environment. A key interest of our lab is to understand this interplay between cancer cell metabolism and tumor evolution, and how we may exploit it for treatment.
An important alteration in cancer cell metabolism is a focus on increased glycolysis, which allows energy production even in the absence of oxygen. Glycolysis is inefficient at producing energy, and as a by-product it acidifies the cell’s environment. Yet it is the main source of energy for many advanced cancers, even in the presence of oxygen - a phenomenon known as the Warburg effect. Together with the Gatenby and Gillies labs, we are exploring the hypothesis that the acid is an important driver of tumor invasion. Using a cellular automaton model we have shown that acid adaptation likely precedes the up-regulation of acid production (Robertson-Tessi, 2015), and that the presence of a glycolytic tumor phenotype is linked to tumor invasion in mouse models (Ibrahim-Hashim, 2017).
Another area in which tumor metabolism plays an important role is in the interaction between drug sensitive and drug resistant cells. Because resistant cells have to divert energy towards the resistance mechanism (e.g. production of drug pumps like p-glycoprotein) they have less energy available for proliferation. This hypothesis has motivated the idea of adaptive therapy, which has proven successful in pre-clinical experiments as well as pilot clinical trials (link to the adaptive therapy part of the site). But does a cost to resistance always exist? And if not, does it matter? To answer this question, we are investigating the role of such costs to resistance for the success of adaptive therapy, and are investigating the metabolism of resistant cells in the wet-lab.